4/2025 | TAE Life Sciences
TAE Life Sciences Partners with alphaXRT to bring Boron Neutron Capture Therapy to Australia and New Zealand
TAE Life Sciences has announced a distribution agreement with alphaXRT, a premier provider of advanced radiation therapy technologies. Under this partnership, alphaXRT will distribute TAE Life Sciences’ Alphabeam™ BNCT system and novel boron drugs, while also providing associated services across Australia and New Zealand.
11/2024 | TAE Life Sciences
TAE Life Sciences and Kyoto University Achieve Breakthrough Preclinical Results in Boron Neutron Capture Therapy (BNCT) with Promising Implications for Cancer Treatment
TAE Life Sciences, in collaboration with Kyoto University and Principal Investigator Dr. Fuyuhiko Tamanoi, is thrilled to announce groundbreaking preclinical results in Boron Neutron Capture Therapy (BNCT). Utilizing TAE Life Science’s novel boron-10 drugs and Kyoto University Research Reactor (KURR) neutron source has generated significant pre-clinical data that may redefine the potential of BNCT in cancer treatment.
TAE Life Sciences and Stella Pharma Announce Strategic Agreement for Development and Commercialization of BPA for BNCT Cancer Therapy in the USA and Europe
TAE Life Sciences (TLS), a leader in developing boron neutron capture therapy (BNCT) technology and associated innovative boron target drugs, and Stella Pharma, the pioneering developer of boronophenylalanine (BPA) under the product name, Steboronine®, for BNCT, are pleased to announce a strategic collaboration focused on the development, commercialization and expansion of BNCT using BPA in the United States and European markets.
TAE Life Sciences Appoints Commercial Leadership Team and Announces Distribution Deal with Radiosurgery Global
TAE Life Sciences a pioneer in advancing Boron Neutron Capture Therapy (BNCT) for cancer treatment, proudly announces the formation of its new commercial leadership team, dedicated to driving the company’s expansion across the Americas, APAC (Asia-Pacific) and EMEAI (Europe, Middle East, Africa, and India).
TAE Life Sciences’ Neutron Beam System Receives Acceptance Enabling the Start of BNCT Clinical Trials in China
TAE Life Sciences, proudly announces the successful completion of acceptance testing and regulatory registration inspection of its accelerator- based neutron beam system (NBS), marking a significant milestone in BNCT global market adoption.
3/2024 | TAE Life Sciences
TAE Life Sciences Awarded Patent for Borylated Amino Acid Compositions for Boron Neutron Capture Therapy
TAE Life Sciences, a leading innovator in cancer treatment
technologies, today announced that the United States Patent and Trademark Office (USPTO) has granted the company’s U.S. Patent No. US 11,884,688 B2, for its groundbreaking borylated amino acid compositions comprising tyrosine derivatives BTS and BTS(OMe)
TAE Life Sciences is developing a next-generation boron neutron capture therapy for cancer. After recently introducing Alphabeam, a compact and affordable accelerator-based neutron system suitable for hospital settings, the company is now focusing on the development of novel boron drugs with improved tumor selectivity and increased 10B loads.
Bruce Bauer, Ph.D., CEO of TAE Life Sciences. The company is developing boron neutron capture therapy (BNCT) as a new radiation therapy for cancer. A patient is first infused with a non-toxic boron-10 compound, which selectively accumulates in tumor tissue. A neutron beam is then focused on the tumor and the neutrons are captured by the boron and causes emission of alpha radiation particles within the tumor.

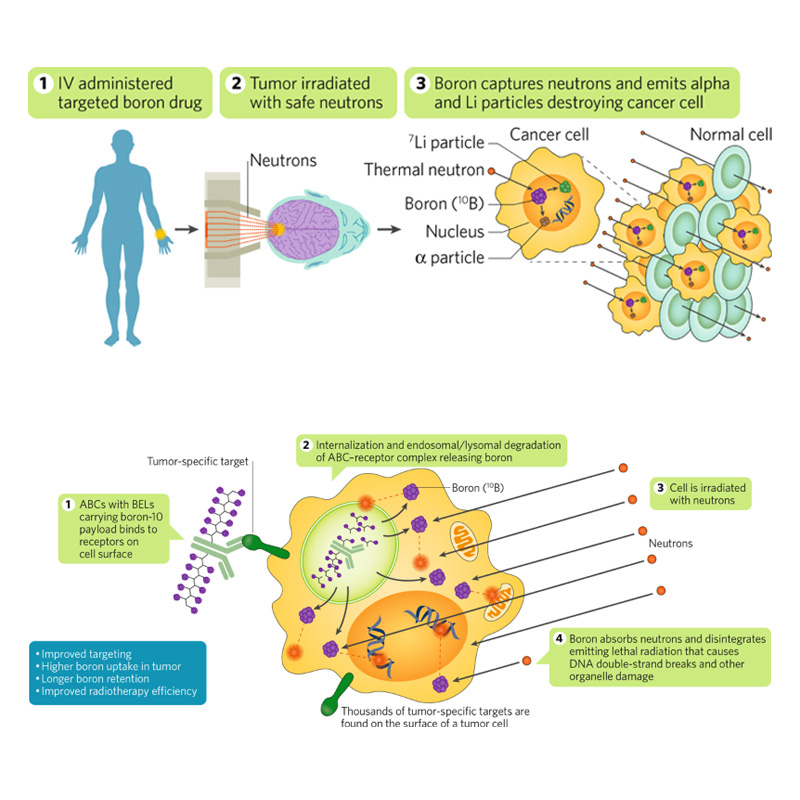
BNCT is a biologically targeted radiation therapy that kills cancer at the cellular level. The technique uses a two-step process. In the first step, patients are injected with a tumor-targeting drug that contains non-toxic and non-radioactive boron-10.

HEALTHCARE: SPIN-OFF FROM FUSION COMPANY AIMING BIG

Nanotechnology is the application of extremely small things—a nanometer is one billionth of a meter—used in science, engineering and technology. It involves the ability to view and control individual atoms and molecules and has been used in designing new therapeutics and diagnostics in medicine. As such, interest is mounting to harness nanotechnology’s potential to enhance radiation therapy and advance cancer care.

BNCT may provide a new option for patients with GBM and head and neck cancers due to its ability to leave healthy tissue unharmed.

TAE Life Sciences, a medical technology company developing an accelerator-based platform for clinical investigation of a promising, previously inaccessible cancer treatment, made its public debut today.

TAE Life Sciences said yesterday it raised $40 million in venture capital to support the development of its neutron beam technology designed as a potential treatment for head and neck, glioblastoma multiforme and other cancers.

Medtech firm TAE Life Sciences is developing a ground-breaking radiotherapy technology which will be able to treat hard-to-reach cancers with targeted beams.

TAE Life Sciences aims to commercialize a cancer radiation treatment called Boron Neutron Capture Therapy. In BNCT, boron-10 is administered to tumors, irradiated with a beam of neutrons, and broken down into charged particles that kill cancer cells.

There’s a radiological medical technique called Boron Neutron Capture Therapy (BNCT), which is like a very targeted chemotherapy.

The neutron beam is aimed at the cancer patient’s tumors for about 30 minutes, and is combined with a boron-based substance that is injected into the patient intravenously and accumulates in the cancer cells.

May 1, 2023 - 7am PST
Online Webinar
Boron Neutron Capture Therapy (BNCT) is a targeted radiation therapy that combines low-energy neutrons with target boron drugs that generate cancer killing alpha and lithium particles. BNCT is going through a renaissance driven by new accelerator-based, in-hospital neutron source technology and novel targeted boron drugs.
Join our complimentary webinar to learn more about the modality and clinical adoption our experts.
Learn more
Have questions about BNCT? Get answers on our new
BNCT Forum















