Developing targeted drugs for boron neutron capture therapy to treat refractory cancers
TAE Life Sciences is developing a next-generation boron neutron capture therapy for cancer. After recently introducing Alphabeam, a compact and affordable accelerator-based neutron system suitable for hospital settings, the company is now focusing on the development of novel boron drugs with improved tumor selectivity and increased 10B loads.
TAE Life Sciences (TLS), founded in 2017, is a privately-held biotechnology company committed to developing next-generation boron neutron capture therapies (BNCTs) to treat patients with invasive, recurrent and difficult to treat cancers.
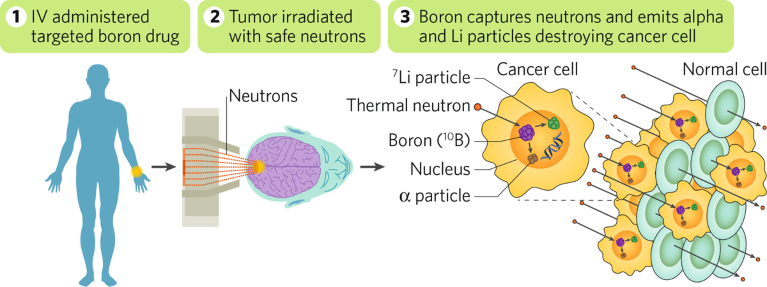
BNCT is best described as a hybrid radiation modality: following infusion of a patient with an inert, non-radioactive, 10B isotope–containing compound that preferentially targets cancer cells, the tumor is irradiated with a low-energy neutron beam that triggers fission of the 10B isotope. The fission reaction releases a high-energy α–particle that causes intracellular double strand DNA breaks leading to apoptosis. By ‘homing’ the lethal effect of radiation to only those cells that have internalized the 10B isotope–containing compound, BNCT minimizes damage to surrounding healthy, non-tumor tissues. To date, approximately 2,000 patients worldwide have undergone BNCT and shown favorable clinical outcomes in glioblastomas, head and neck, and other challenging cancers (Fig. 1).
Fig. 1 | TAE Life Sciences’ next-generation BNCT for cancer. Following infusion with an inert, non-radioactive, 10B isotope–containing compound that preferentially targets cancer cells, the patient is irradiated with a low-energy neutron beam that triggers fission of the 10B isotope in tumor cells. The fission reaction releases a high energy α–particle that causes apoptosis in only those cells that have internalized the 10B isotope–containing compound.
Broader implementation of BNCT, however, has been hampered by (1) limited access to neutron sources—until recently only available at non-hospital settings such as at nuclear research reactors—and (2) the limited effectiveness of current 10B isotope–containing compounds—the most advanced boron drug available is boronophenylalanine (BPA), a ‘passive’ drug that relies exclusively on the higher amino acid uptake rates of rapidly dividing tumor cells compared with non-tumor cells.
The introduction over the past decade of compact and affordable accelerator-based neutron sources well suited for a hospital setting has now removed the physical barrier to a wider adoption of BNCT and triggered a renewed interest in the development of novel 10B-containing compounds of improved target selectivity and increased boron load.
TLS has established a comprehensive approach to the development of solutions that will enable the implementation of next-generation BNCT as a first-line treatment for cancer patients. On the neutron source front, the company’s Alphabeam Neutron System (ANS), a positive ion-based electrostatic accelerator device, will provide the most compact, reliable and economical solution for the clinical implementation of BNCT. On the boron drug front, TLS is advancing a pipeline of novel boron drugs for the US and European markets that include, boronated amino acid and small peptide alternatives to BPA, antibody boron conjugates (ABCs), and boron enriched nanoparticles.
The company is collaborating with leading cancer research centers globally to deploy the ANS and establish clinical BNCT facilities, at the same time securing partnerships with key academic and clinical institutions around the world to drive the research of novel boron drugs for improved tumor selectivity and delivery of exponentially higher 10B loads to the target cells than current drugs. In-house integration of both efforts allows for the engineering of optimal and complete BNCT systems.
“At TLS we have assembled a world-class, cross-functional team of clinicians, radiation oncologists, physicists, and other researchers to bring BNCT to cancer patients with the most aggressive and recurrent cancers,” said Bruce Bauer, CEO of TLS. “With in-hospital access to a neutron source now becoming a reality, TLS is making a significant investment in developing a portfolio of new boronated compounds to help expand the application of BNCT to new cancer types and to provide even better outcomes for indications historically treated with BPA.”
Get a deeper look at this critical frontier of cancer treatment by downloading a new white paper: Improving Cancer Treatment with Biologically Targeted, Tumor-Specific Particle Therapy. The paper looks closely at how particle therapies hold transformative potential to, both literally and metaphorically, treat cancer cases at the margins—and how the path to mainstream adoption of BNCT is taking shape.
Next generation boron drugs
In March 2020, the Japanese Pharmaceuticals and Medical Devices Agency approved the first ever BNCT system, consisting of a medical-grade accelerator, a dose calculation program and a treatment planning platform. This was shortly followed by the approval of BPA for the treatment of advanced and recurrent head and neck cancer, and a June ruling by the Japanese National Health Insurance allowing reimbursements for BNCT.
Against this backdrop, TLS is now expediting the commercialization of the intravenous formulation of BPA, for approval in the US and in Europe. In parallel, TLS is also readying its ANS for regulatory submission both in the US and in Europe, with a target date for first installations of 2022. The initial target indications for TLS’ BNCT system will be advanced and recurrent head and neck cancer, gliomas, and melanomas—all cancers treated historically with BPA. The goal is to start clinical trials in the US and in Europe in the next 2 to 3 years.
Although clearly effective and an excellent first-generation boron drug for BNCT, BPA has limitations in achieving the levels of tumor cell targeting and 10B delivery necessary for therapeutic application across a broad range of cancer types. To achieve the full potential of BNCT, improved boron-carrying drugs need to be developed. TLS is already working toward this goal by designing more effective next generation boron drugs that address the fundamental limitations of BPA.
First, and in order to maximize the effectiveness of BNCT, the 10B load per cell must be improved. Current estimates are that a boronated drug should deliver at least 20 μg of 10B per gram of tumor. With BPA carrying only one boron atom per molecule, there is room to improve the cellular load by augmenting the number of atoms per boron drug molecule internalized.
Second, the specificity of the boronated compounds for tumor cells has to be maximized. The minimal tumor to healthy cell boron ratio for a successful BNCT is 3:1. With BPA uptake being determined solely by the enhanced metabolic needs of cancer cells, the typical ratios achieved with it hover around that lower range, leaving room for improvement by increasing the selectivity of the boron drugs for cancer cells.
While the short path length of the high energy α–particles generated following the fission reaction guarantees their destructive effect is kept within the cellular confines—making BNCT a safe therapeutic option—efficacy of the method relies on the ability to deliver the boronated compounds to all or as many tumor cells as possible. While BPA performs well in terms of diffusion into tumors, a variety of medicinal chemistry tools will help improve intratumoral diffusion and optimize dosing of novel boron drugs.
TLS is addressing all of these issues with a pipeline of diverse drug development approaches supported by a number of different patented molecular designs and scalable synthetic routes. Initial results are already showing superior 10B delivery compared to existing 10B carriers.
Small molecules
Small molecules are a way to treat many diseases including cancer. In particular, small molecules are well suited for the treatment of brain tumors because the size of the compounds enables them to cross the blood–brain barrier and engage targets in the brain. TLS is developing novel boronated amino acids and other small molecules of increased solubility, improved cell uptake and retention and easier formulation than BPA. The company’s lead compound is TDP-747, an amino acid analogue that is taken up by the large neutral amino acid transporter (LAT-1), which is upregulated in many cancers. In vivo, TDP-747 shows a tumor to healthy cell boron ratio of 13:1, a vast improvement over the 3:1 ratio usually seen with BPA.
Antibody boron conjugates
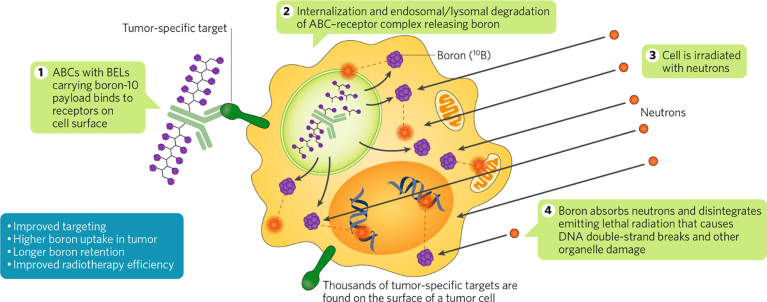
To address the issue of specificity, TLS is leveraging some antibody–drug conjugate (ADC) technology to develop antibody boron conjugates (ABCs) for improved differential penetration of boron drugs into tumor cells. ABCs consist of an antibody moiety and a boron-containing payload. Unlike small molecules such as amino acids, ABCs are designed to specifically target tumor cells and they have a longer serum half-life. To facilitate ABC development, TLS is developing boron enriched linkers (BELs) capable of carrying large 10B loads. These linkers can be modified for size, charge, stability, and branching (Fig. 2). In proof-of-concept studies, TLS has succeeded to generate loads of up to 400 boron atoms per antibody using lysine conjugation and BELs. Such high-load ABCs should help improve the efficacy of BNCTs, as well as expand the spectrum of cancer indications treatable with BNCT.
Fig. 2 | Antibody boron conjugate with BELs. Traditionally, boron neutron capture therapies (BNCT) have relied on the incorporation of boronophenylalanine in tumor cells due to their higher amino acid uptake rates compared with non-tumor cells. TLS’ novel approach relies on the use of antibodies loaded with boron-enriched linkers (BELs) with the goal to improve targeting and increase both specificity for tumor cells and boron-loading of the cells for BNCT. ABC, antibody boron conjugate.
Nanoparticles
In collaboration with Kyoto University, TLS is also developing a novel class of nanoparticle carriers called BPMOs (biodegradable mesoporous organosilica nanoparticles) that can carry large 10B loads and deliver them with high selectivity to cancer cells. Nanoparticles of the size being made under this collaboration have been shown to home to tumor cells as a result of what is known as an EPR effect, enhanced penetration and retention. Chick embryo studies with BPA-loaded BPMOs have already shown high efficacies and specificities, pointing to the potential of these nanoparticles for BNCT. In vivo studies in animal models are now planned.
“The approval of the first complete BNCT platform last year in Japan signaled the start of a new era for radiation-based treatment of cancer,” said Bauer. “At TLS we are committed to expanding the application of BNCT to a broader list of cancer types, and to improve outcomes for indications historically treated with BPA, through the development of a portfolio of novel targeted boron drugs that will help increase the safety and efficacy of BNCT exponentially.”
Partnering BNCT
TLS has a mission to develop BNCTs to help improve the lives of patients with invasive, recurrent and difficult to treat cancers. Because BNCT represents the sum of multiple technologies, TLS has adopted a collaborative strategy to allow it to incorporate the most advanced solutions for each technology involved in BNCT—from the neutron source to the tumor cell targeting moiety.
TLS has several agreements in place to install its ANS in different locations around the world and is establishing a network of collaborations to develop other components of the BNCT system.
“The ongoing BNCT renaissance has been fueled mostly by the technological advances on the neutron source front,” said Bauer. “New windows of opportunity have now opened for the development of novel boron drugs to capitalize on the potential of BNCT, and we believe this will be best achieved through collaborative approaches that keep patients and their wellbeing as a main focus.”
The TLS’ Alphabeam Neutron System and boron drugs are in development and are not available commercially.