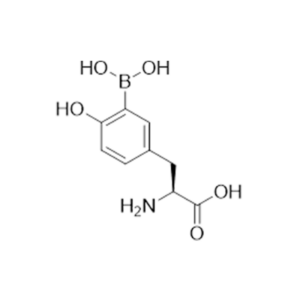
TAE Life Sciences has today announced a landmark agreement with Biddle Sawyer to serve as the exclusive supplier of TC220, a novel boronated amino acid analog drug for boron neutron capture therapy (BNCT). As part of this exclusive agreement, Biddle Sawyer has enlisted contract research and manufacturing organization Kinentia Biosciences to refine and streamline the drug's manufacturing process, and ultimately produce the drug in their state-of-the-art GMP facility. Kinentia Biosciences will be the exclusive manufacturer of TC220 for the US market.
Press Releases
Rob Hill Promoted to CEO of TAE Life Sciences as Company Pursues Global Clinical Trials
Hill brings decades of global leadership in the development of innovative cancer care solutions, corporate operations, sales, and fundraising experience to the role
IRVINE, Calif., July 25, 2023 – The board of directors of TAE Life Sciences, a pre-clinical stage biotech company focused on advancing the field of radiation oncology through accelerator-based Boron Neutron Capture Therapy (BNCT), is pleased to announce the appointment of Rob Hill as its new Chief Executive Officer (CEO). Effective immediately, Hill succeeds Bruce Bauer who has served as CEO since the company was founded in 2017. Bauer, stepping aside for medical reasons, will remain active on the board of directors as chairman emeritus.
The Board of Directors is grateful to Bauer for his significant contributions as CEO. Under his leadership, TAE Life Sciences licensed its accelerator technology from TAE Technologies, the world's largest and most advanced private fusion energy company, secured Series A and Series B funding, developed and deployed its first neutron beam system, arranged strategic partnerships with world-renowned cancer centers for clinical trials, and envisioned and established a world-class pharmaceutical development team.
With an impressive track record in the radiation oncology industry and extensive experience in corporate strategy, product development, and global market expansion, Hill brings invaluable expertise to his new role as CEO of TAE Life Sciences. His deep understanding of the industry, coupled with his visionary leadership, will play a pivotal role in propelling the company forward as it enters the critical clinical trial phase for its groundbreaking Alphabeam™ BNCT system with its novel targeted boron drugs.
Having joined TAE Life Sciences as Chief Operating Officer in October 2018, Hill has already made significant contributions to the company's growth and success. Under his leadership, TAE successfully delivered its neutron beam system and accelerator to its joint-venture partner in China, overcoming many challenges posed by the global pandemic. He also played a crucial role in securing partnerships with prominent medical institutions and medical distribution partners from around the world, growing the company’s R&D organization, and fundraising for the company.
"I am truly honored to lead TAE Life Sciences, a company poised to revolutionize cancer treatment and save countless lives,” said Hill. “I’m confident that our Alphabeam BNCT machine, combined with our proprietary targeted drugs, will profoundly impact the lives of patients worldwide. With our exceptional team, we'll bring this groundbreaking therapy to market, and make a meaningful difference in the fight against cancer."
Before joining TAE Life Sciences, Hill held various senior executive positions at Accuray Incorporated, a renowned provider of radiation oncology solutions. During his tenure, he led the development and launch of innovative products, drove strategic partnerships, and played a crucial role in transforming the company's product development organization. His accomplishments at Accuray include launching new radiation therapy products, intellectual property development, and driving significant revenue growth.
Under Hill’s leadership, TAE Life Sciences is well-positioned to achieve its strategic goals, commence clinical trials globally, and become a global leader in the radiation oncology market with its Alphabeam BNCT system and novel targeted boron drugs.
For more information about TAE Life Sciences, Alphabeam, and the company’s proprietary boronated BNCT drugs, please visit taelifesciences.com.
About TAE Life Sciences
TAE Life Sciences is a privately held biotechnology company committed to developing a new biologically targeted radiation therapy based on Boron Neutron Capture Therapy (BNCT). TAE Life Sciences is the only company developing the next-generation targeted boron drugs and low-energy accelerator-based neutron system optimized for an in-hospital BNCT program that delivers cancer-killing radiation with cellular-level precision to treat patients with aggressive and refractory cancers. TAE Life Sciences’ Alphabeam neutron system and targeted boron drugs are currently in development and have not been approved for sale. More information about TAE Life Sciences is available at taelifesciences.com.
Preliminary Results Show TAE Life Sciences’ Novel Boron-Containing Drug Holds Promise for Revolutionizing Boron Neutron Capture Therapy
Andrea Monti Hughes, PhD presented at 61st Annual PTCOG Conferences, in vivo experimental testing data of novel boronated mediated Boron Neutron Capture Therapy
Madrid and Irvine, Calif., July 06, 2023 – TAE Life Sciences, a pioneering company in the field of cancer treatment, today announces the results from early testing of its TC440 boron-containing dipeptide compound for Boron Neutron Capture Therapy (BNCT). The findings suggest that TC440, along with its counterpart TC442, holds promise in revolutionizing cancer treatment strategies.
"Finding safe and effective ways to treat a variety of cancers is of utmost importance in our mission to improve and transform cancer care. The work we are doing at TAE Life Sciences to create new drugs like TC440 for BNCT is critical in this endeavor,” said Kendall Morrison, Chief Science Officer, TAE Life Sciences. “The promising preliminary results from our early testing indicate that these exciting new peptides hold significant potential in revolutionizing cancer treatment strategies. These findings highlight the immense potential of TC440 in enhancing the therapeutic efficacy of BNCT, bringing us closer to a future where safer and more effective treatments are available for cancer patients.”
TC440 and TC442 belong to a family of dipeptides that were synthesized at TAE Life Sciences using cuttingedge BPA Structural Analysis Relationship (SAR) chemistry. These compounds have demonstrated superior solubility compared to the widely used Boronophenylalanine (BPA), enabling the formulation of highly concentrated solutions. Formulations of TC440 and TC442 in fructose at 150 mg/ml have been achieved, providing a remarkable advancement in delivering boron to tumors. By leveraging the dipeptides' improved solubility, higher tumor boron delivery was achieved in multiple xenograft models, showcasing the superior uptake of TC440 and TC442. In addition to enhanced solubility, TC440 and TC442 possess the ability to be internalized via LAT1 transporters and PEPT1, even in LAT1 negative cell lines.
One of the most exciting discoveries is that these compounds deliver two to three times more boron than BPA in multiple human xenograft models and have a longer half-life compared to BPA. This increased boron delivery capability and longer half-life hold immense potential in enhancing the therapeutic efficacy of BNCT as a treatment modality.
To further investigate the therapeutic applications of TC440 and TC442, TAE Life Sciences is collaborating on BNCT experiments with the Department of Radiobiology at the National Atomic Energy Commission (CNEA) with Dr. Andrea Monti Hughes and Team, from Argentina. Initial studies conducted in the hamster cheek pouch oral cancer model have yielded encouraging preliminary results and were presented at 61st Annual PTCOG Conferences held in Spain, this year.
The ongoing study conducted in the hamster cheek pouch oral cancer model, which closely mimics the development of precancer and malignant human oral tumors, is a critical milestone in the research journey. The model provides a unique tumor environment surrounded by precancerous tissue, allowing researchers to investigate BNCT's dose-limiting effects, as observed in field-cancerized oral mucosa in head and neck cancer patients.
In this preliminary study, an 800 mg TC440/kg dose has demonstrated an increase in boron uptake compared to the 300 mg BPA/kg dose typically administered during testing, while maintaining higher tumor/blood and tumor/normal tissue ratios. However, similar boron concentrations and ratios were achieved compared to 800 mg BPA/kg. Unlike the BPA solution, the TC440 formulation exhibited no precipitation issues, providing a practical advantage.
When evaluating the therapeutic effect of BPA/BNCT versus TC440/BNCT, the preliminary study unveiled promising results. TC440 demonstrated a doubling of complete responses compared to BPA/BNCT protocols, particularly in medium and large hamster tumors treated with TAE/BNCT. Additionally, partially responded tumors treated with TC440 that exhibited a 50% in volume reduction were higher than in the BPA/BNCT group. Ongoing studies to confirm these early findings are underway.
Ongoing studies will further explore the potential of new dipeptides from TAE Life Sciences and expand upon these exciting preliminary findings. “The study of new boron compounds that could increase significantly BNCT therapeutic effect while preserving dose-limiting tissues is of utmost importance in BNCT. Huge efforts have been done for many years around the World, and radiobiological studies in in vivo models are a critical step to decide if a boron compound could be consider potentially useful in a clinical setting” said Andrea Monti Hughes (CNEA/CONICET, Argentina).
The groundbreaking discovery of TC440 and its therapeutic potential for cancer treatment using BNCT marks a significant milestone in the field of oncology. The company will continue to investigate the therapeutic potential of its TC440 and TC442 boron-containing drugs and will have the results published in a peer-reviewed scientific journal.
TAE Life Sciences is committed to advancing innovative solutions to address the challenges of cancer treatment, with the goal of improving patient outcomes and transforming the future of cancer care. Morrison will be presenting the results of this collaborative study with CNEA at the 19th Annual Japanese Neutron Capture Therapy Congress in Kyoto, Japan.
For more information about TAE Life Sciences, Alphabeam, and the company’s proprietary boronated BNCT drugs, please visit taelifesciences.com.
About BNCT
BNCT is a combination treatment based on the reaction that occurs when a non-toxic compound containing boron-10 is irradiated with a low-energy neutron beam. BNCT differs radically from other radiation therapies and shows promise in becoming the next-generation cancer treatment. Research has shown that BNCT has the capability of killing cancer cells that are resistant to traditional radiation therapy with limited harm to healthy tissue. Current advances in both neutron radiation technology and medicinal boron drug targeting are enabling BNCT’s potential to improve patient care while also improving treatment economics. To date, approximately 2,000 patients have been treated with BNCT at research sites worldwide.
About TAE Life Sciences
TAE Life Sciences is a privately held biotechnology company committed to developing a new biologically targeted radiation therapy based on Boron Neutron Capture Therapy (BNCT). TAE Life Sciences is the only company developing the next-generation targeted boron drugs and low-energy accelerator-based neutron system optimized for an in-hospital BNCT program that delivers cancer-killing radiation with cellular-level precision to treat patients with aggressive and refractory cancers. TAE Life Sciences’ Alphabeam neutron system and targeted boron drugs are currently in development and have not been approved for sale. More information about TAE Life Sciences is available at taelifesciences.com.
Leading Cancer Center in Poland Announce Plans for a New State-of-the-Art BNCT Facility
Construction of the facility is projected to be completed by 2025, with the first clinical trials scheduled to commence in spring 2026
Irvine, Calif., and Bydgoszcz, Poland, June XX, 2023 – TAE Life Sciences, a global leader in advanced biological-targeted radiation therapy, and creator of next-genera on boron neutron capture therapy (BNCT) systems, and the Franciszek Łukaszczyk Oncology Center (FLOC) are proud to announce their collaboration to establish a cu ng-edge Boron Neutron Capture Therapy (BNCT) Center in Bydgoszcz, Poland. This groundbreaking radiation therapy facility will utilize TAE Life Sciences’ AlphaBeam® BNCT system to provide targeted treatment to patients with particularly challenging forms of cancer, including brain tumors.
BNCT is an innovative radiotherapy technique that offers a significant advantage over conventional methods by minimizing the negative effects on healthy tissues. By selectively targe ng cancer cells while preserving surrounding healthy tissue, BNCT shows immense promise in improving patient outcomes.
“We are honored to be the second center in Europe to implement the TAE Life Sciences BNCT groundbreaking treatment method,” emphasized Marshal Piotr Całbecki. “For many patients, this represents a chance for life-saving treatment and renewed hope.”
During the signing ceremony held on June 16th, Prof. Janusz Kowalewski, the director of the Franciszek Łukaszczyk Oncology Center, highlighted the importance of this modern and safe therapy. “BNCT is already successfully used in Japan, China, and Taiwan, and soon it will be introduced in Italy as well. FLOC’s partnership with TAE Life Sciences is a testament to Poland’s commitment to providing exceptional healthcare. Furthermore, this new center will greatly enhance our center’s scientific potential.”
The future BNCT Center at the Franciszek Łukaszczyk Oncology Center will serve as a leading research and therapeutic facility, conducting meticulous clinical trials. By joining the elite global network of BNCT centers, FLOC will initially focus on treating the most challenging malignant brain, head, and neck tumors, with plans to expand its scope to other types of cancer. The construction of the facility is projected to be completed by 2025, with the first clinical trials scheduled to commence in spring 2026.
This collaborative endeavor between TAE Life Sciences and FLOC represents a significant step forward in cancer treatment and demonstrates the commitment to advancing medical innovation in Poland. Together, they aim to provide a new ray of hope for patients battling the most challenging forms of cancer.
For more information about TAE Life Sciences, Alphabeam, and the company’s proprietary boronated BNCT drugs, please visit taelifesciences.com.
About TAE Life Sciences
TAE Life Sciences is a privately held biotechnology company committed to developing a new biologically targeted radiation therapy based on Boron Neutron Capture Therapy (BNCT). TAE Life Sciences is the only company developing the next-generation targeted boron drugs and low-energy accelerator-based neutron system optimized for an in-hospital BNCT program that delivers cancer-killing radiation with cellular-level precision to treat patients with aggressive and refractory cancers. TAE Life Sciences’ Alphabeam neutron system and targeted boron drugs are currently in development and have not been approved for sale. More information about TAE Life Sciences is available at taelifesciences.com.
About FLOC
The Franciszek Łukaszczyk Oncology Center (FLOC) in Bydgoszcz is a modern hospital at the forefront of cancer treatment in Poland. It is renowned for its exceptional care, continuous modernization efforts, and commitment to scientific advancement. FLOC is managed by the local government of the Kujawsko-Pomorskie voivodeship and is part of a broader healthcare network serving the region.
TAE Life Sciences Announces First Cancer Patient Treatments Using Accelerator-Based Biologically Targeted Radiation Therapy (BNCT)
2023 | Business Wire | Read Now
TAE Life Sciences (TLS), a biological-targeted radiation therapy company developing next-generation boron neutron capture therapy (BNCT), today announced that the first accelerator-based BNCT (AB-BNCT) clinical research center at Xiamen Humanity Hospital (XHH) has now treated 12 patients in its Investigator Initiated Trial (IIT) aimed at evaluating the safety and efficacy of BNCT in the treatment of advanced refractory malignant tumors. Treated with the NeuPex System from Neuboron with TLS’s electrostatic Neutron Beam System (NBS), these are the first human patients treated with AB-BNCT technology outside of Japan.
TLS and Avita Biomedical Announce Strategic Partnership
2023 | Business Wire | Read Now
TAE Life Sciences (TLS), a biological-targeted radiation therapy company developing next-generation boron neutron capture therapy (BNCT), and AIVITA Biomedical, Inc. (AIVITA), a biotech company specializing in innovative stem cell applications, today announced a partnership to address an unmet need in glioblastoma treatment.
TLS Announces Partnership with HDX for BNCT in South Korea
2022 | Business Wire | Read Now
TAE Life Sciences (TLS), a biological-targeted radiation therapy company developing next-generation boron neutron capture therapy (BNCT), today announced a partnership with HDX Corporation (HDX) to bring TLS’s targeted radiation therapy to South Korea for the treatment of difficult-to-treat cancers, such as brain, head and neck cancers and melanoma.
TAE Life Sciences Sponsored Study Details Results of Proton Boron Fusion Therapy Biological Effectiveness
2022 | Business Wire | Read Now
TAE Life Sciences (TLS), a biological-targeted radiation therapy company developing next-generation boron neutron capture therapy (BNCT), today announced the publication of a study in the September 22 issue of the Journal of Medical Physics that assessed whether alpha particles generated through the combination of boron target drugs and proton radiation would generate a reaction that would be sufficient to enhance the biological effectiveness of proton therapy in the treatment of prostate cancer.
TAE Life Sciences to Sponsor Industry Expert Theater “New Era in Biologically-Targeted Radiation Therapy” at 2021 ASTRO Annual Meeting
10/2021 | Business Wire
The session, “New Era in Biologically-Targeted Radiation Therapy: Clinical Application and Technical Advancement in Boron Neutron Capture Therapy” will feature presentations by Timothy Malouff, M.D., Mayo Clinic, Ester Orlandi, M.D., National Center of Oncological Hadrontherapy (CNAO) and Kendall Morrison, Ph.D., Chief Scientific Officer, TAE Life Sciences. They will discuss the clinical application and technical advancements in BNCT for the treatment of invasive, recurrent and difficult to treat cancers.